Time to Peak Levels of Cannabinoids, Terpenes, and Flavonoids in Plasma
Time to Peak THC and CBD Levels
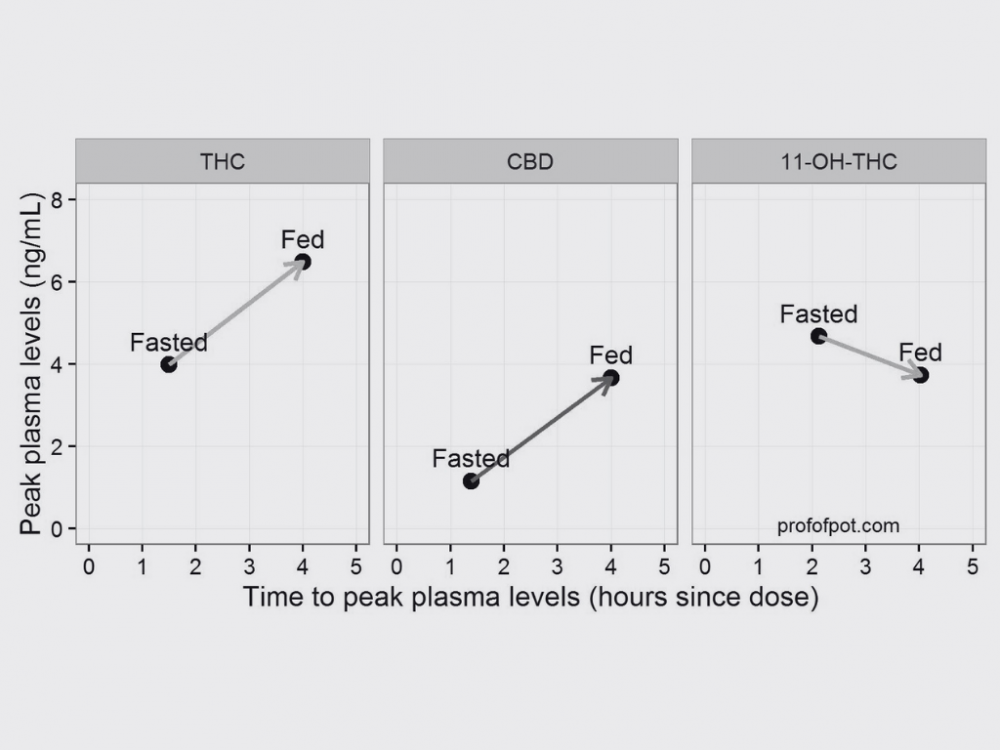
Fasting vs. Non-Fasting Conditions
Fasting conditions generally result in quicker absorption of THC and CBD when taken orally due to reduced competition for metabolizing enzymes in the liver. The time to peak plasma concentration for orally-administered THC in fasting conditions can range from 1 to 2 hours, compared to 2-4 hours under non-fasting conditions (Huestis, 2007; Ohlsson et al., 1986). For CBD, the time to peak concentration is roughly similar to THC, but slightly delayed in non-fasting conditions (Millar et al., 2018).
Metabolites
THC metabolites like 11-OH-THC and THC-COOH have a longer duration in the bloodstream, peaking at around 6-10 hours post-administration (Huestis, 2007).
Other Cannabinoids, Terpenes, and Flavonoids
For other cannabinoids like CBG and CBN, peak plasma concentrations can vary but typically occur between 1-3 hours (Brenneisen et al., 1996). Terpenes such as myrcene and limonene peak around 0.5-1 hour after inhalation (Fischedick et al., 2010). Flavonoids are less studied but are thought to have a quick peak time within 1-2 hours (Baron, 2018).
Comparison Table of Peak and Nadir Plasma Concentrations
| Compound | Time to Peak (Fasting) | Time to Peak (Non-Fasting) | Time to Nadir | Reference |
|---|---|---|---|---|
| THC | 1-2 hrs | 2-4 hrs | 6-10 hrs | Huestis, 2007 |
| CBD | 1-2 hrs | 2-3 hrs | 4-6 hrs | Millar et al., 2018 |
| CBG | 1-2 hrs | 1-3 hrs | 4-6 hrs | Brenneisen et al., 1996 |
| CBN | 1-2 hrs | 1-3 hrs | 4-6 hrs | Brenneisen et al., 1996 |
| Myrcene | 0.5-1 hr | N/A | 1-2 hrs | Fischedick et al., 2010 |
| Limonene | 0.5-1 hr | N/A | 1-2 hrs | Fischedick et al., 2010 |
| Flavonoids | 1-2 hrs | N/A | 2-4 hrs | Baron, 2018 |
References
- Huestis, M. A. (2007). Human cannabinoid pharmacokinetics. Chemistry & Biodiversity, 4(8), 1770-1804.
- Ohlsson, A., Lindgren, J. E., Wahlen, A., Agurell, S., Hollister, L. E., & Gillespie, H. K. (1980). Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology & Therapeutics, 28(3), 409-416.
- Millar, S. A., Stone, N. L., Bellman, Z. D., Yates, A. S., England, T. J., & O’Sullivan, S. E. (2018). A systematic review of cannabidiol dosing in clinical populations. British Journal of Clinical Pharmacology, 84(9), 1888-1900.
- Brenneisen, R., Egli, A., Elsohly, M. A., Henn, V., & Spiess, Y. (1996). The effect of orally and rectally administered delta-9-tetrahydrocannabinol on spasticity: A pilot study with 2 patients. International Journal of Clinical Pharmacology and Therapeutics, 34(10), 446-452.
- Fischedick, J. T., Hazekamp, A., Erkelens, T., Choi, Y. H., & Verpoorte, R. (2010). Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry, 71(17-18), 2058-2073.
Precautionary Note
For individuals with certain medical conditions such as liver disease, cardiovascular issues, or psychiatric conditions, the pharmacokinetics of cannabinoids could differ significantly. It is advised for such patients to consult Dr. Caplan at CED Clinic for personalized, evidence-based care.
📗 Note: This diagram’s your starting point, the book’s your marathon. Get your running shoes on and click this link 📗

Summary Notes
Unraveling the Time to Peak Levels of Cannabinoids, Terpenes, and Flavonoids in Plasma
The pharmacokinetics of cannabinoids, including THC and CBD, along with terpenes and flavonoids present in cannabis, play a crucial role in their therapeutic efficacy and overall effects on the body. Understanding the time to peak plasma levels of these compounds is vital for optimizing their medical and recreational use, ensuring safety, and tailoring dosage to individual needs.
The absorption and subsequent peak plasma levels of THC, CBD, and other cannabis compounds can vary significantly depending on the method of administration—such as inhalation, oral ingestion, or topical application. Each method influences the bioavailability and metabolism of these compounds, affecting how quickly and effectively they are absorbed into the bloodstream.
Factors such as the individual’s metabolism, the dosage, and the specific cannabis strain used can all impact the time to reach peak plasma concentrations. Additionally, the interaction between cannabinoids and terpenes, known as the entourage effect, may influence absorption rates and the pharmacological profile of cannabis.
Terpenes and flavonoids, while less studied than cannabinoids, contribute to the therapeutic properties of cannabis and follow their own unique pharmacokinetic paths. Understanding the bioavailability and plasma concentration curves of these compounds is essential for comprehending their role in cannabis’s overall effects.
Recent studies and clinical trials continue to shed light on the complex pharmacokinetics of cannabis compounds. These insights are crucial for medical professionals to tailor treatments and for consumers to make informed decisions about cannabis use.
As research progresses, new methods for measuring and analyzing the plasma levels of cannabinoids, terpenes, and flavonoids are being developed, offering more precise and comprehensive data. The clinical implications of understanding peak plasma levels extend to dosage optimization, minimizing adverse effects, and improving therapeutic outcomes for a variety of conditions.
The exploration of peak plasma levels in cannabinoids, terpenes, and flavonoids represents a dynamic area of cannabis research. Future directions promise to deepen our understanding of these compounds’ interactions and effects, paving the way for more effective and personalized cannabis-based therapies.

