Transformation & Metabolism of Delta-9 THC: A Chemistry Perspective
Delta-9-tetrahydrocannabinol (D9-THC), the main psychoactive compound in cannabis, undergoes a complex metabolic process in the body. This involves enzymatic conversion into various metabolites, each with their distinct biological activity and receptor affinity. Understanding the kinetics and pathways involved in the metabolism of D9-THC helps clarify its duration of action, efficacy, and safety profile.
Transformation and Metabolism of D9-THC
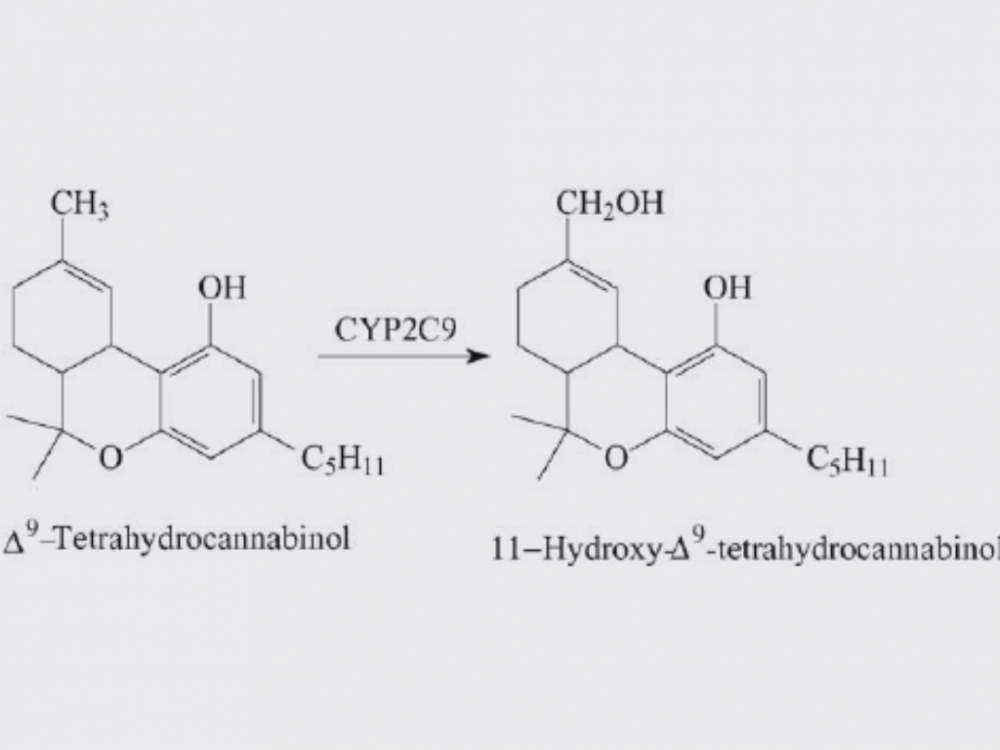
After consumption, D9-THC is primarily metabolized in the liver by cytochrome P450 enzymes, mainly CYP2C9 and CYP3A4, into 11-hydroxy-THC (11-OH-THC) and further into 11-nor-9-carboxy-THC (THC-COOH). 11-OH-THC is psychoactive and has similar effects to its parent compound. In contrast, THC-COOH is inactive but serves as a long-term marker for cannabis consumption (Huestis, 2007). These metabolites can then be excreted via urine and feces (Grotenhermen, 2003).
Receptor Binding
D9-THC and 11-OH-THC primarily interact with CB1 receptors located in the central nervous system, thereby eliciting psychoactive effects. On the other hand, THC-COOH has negligible affinity for these receptors and is considered inactive (McPartland et al., 2015).
Timing
The onset of effects from D9-THC is rapid, usually within minutes when smoked and within 30 minutes to 2 hours when ingested. However, the metabolites can remain detectable for days to weeks, particularly THC-COOH, which is stored in fat tissues (Huestis, 2007).
Factors Affecting Metabolism
- Induction: St. John’s Wort, a herbal supplement, can induce CYP3A4, potentially accelerating THC metabolism.
- Inhibition: Grapefruit juice inhibits CYP3A4, potentially prolonging the duration of THC effects.
Comparison Table: Compounds of THC, Effects, and Methods of Breakdown
| Compound | Psychoactive Effects | Receptor Affinity | Method of Breakdown | Time to Excrete |
|---|---|---|---|---|
| D9-THC | High | CB1, CB2 | CYP2C9, CYP3A4 to 11-OH-THC | Minutes to Hours |
| 11-OH-THC | High | CB1 | Further metabolized to THC-COOH | Hours to Days |
| THC-COOH | None | Negligible | Excreted in urine and feces | Days to Weeks |
Medical Illnesses and Diagnoses
Patients with the following medical conditions should exercise caution when considering the use of cannabinoids:
- Liver Diseases
- Cardiovascular Diseases
- Psychiatric Disorders
For those falling under these categories, consultation with Dr. Caplan at CED Clinic is highly advised for specialized and informed cannabis care.
References:
- Huestis, M. A. (2007). Human Cannabinoid Pharmacokinetics. Chemistry & Biodiversity, 4(8), 1770–1804.
- Grotenhermen, F. (2003). Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical Pharmacokinetics, 42(4), 327–360.
- McPartland, J. M., Duncan, M., Di Marzo, V., & Pertwee, R. G. (2015). Are cannabidiol and Δ9‐tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. British Journal of Pharmacology, 172(3), 737–753.
📗 Note: If the diagram was a quick chat, the book’s a deep conversation over a cup of cannabis tea. Brew your thoughts here 📗

Summary Notes
Understanding the Transformation of Delta-9 THC: A Chemistry Perspective
The transformation of Delta-9 tetrahydrocannabinol (THC), the principal psychoactive constituent of cannabis, is a subject of significant interest from a chemistry standpoint. This exploration delves into the molecular intricacies and environmental influences that govern the stability and conversion of Delta-9 THC into other compounds.
Chemically, Delta-9 THC is known for its sensitivity to heat, light, and oxygen, each playing a pivotal role in its transformation. The application of heat can induce decarboxylation, converting THC into its active form, which is essential for its psychoactive effects upon consumption. Conversely, photodegradation under light exposure can lead to the breakdown of Delta-9 THC, significantly altering its potency and effects.
The process of oxidation, whether enzymatic within the body or chemical in the environment, can transform Delta-9 THC into cannabinol (CBN), a less psychoactive cannabinoid. This transformation is of particular interest for understanding the shelf life and potency of cannabis products.
The stability of Delta-9 THC is also influenced by factors such as pH, solubility, and the presence of solvents, which can affect its bioavailability and therapeutic efficacy. Additionally, the isomerization process can convert Delta-9 THC into other cannabinoids, further expanding the spectrum of cannabis’s pharmacological properties.
Analytical methods play a crucial role in monitoring these chemical transformations, offering insights into the kinetics and mechanisms underlying Delta-9 THC’s stability and degradation. Such knowledge is vital for the development of storage and preservation techniques aimed at maintaining the integrity of Delta-9 THC in cannabis products.
As research continues to advance, understanding the chemical behavior of Delta-9 THC remains a dynamic field, promising to unveil new synthetic pathways, therapeutic potentials, and challenges in the preservation of cannabis quality. The intricate chemistry of Delta-9 THC transformation not only enriches our understanding of cannabis but also opens avenues for future innovations in cannabinoid science and technology.

